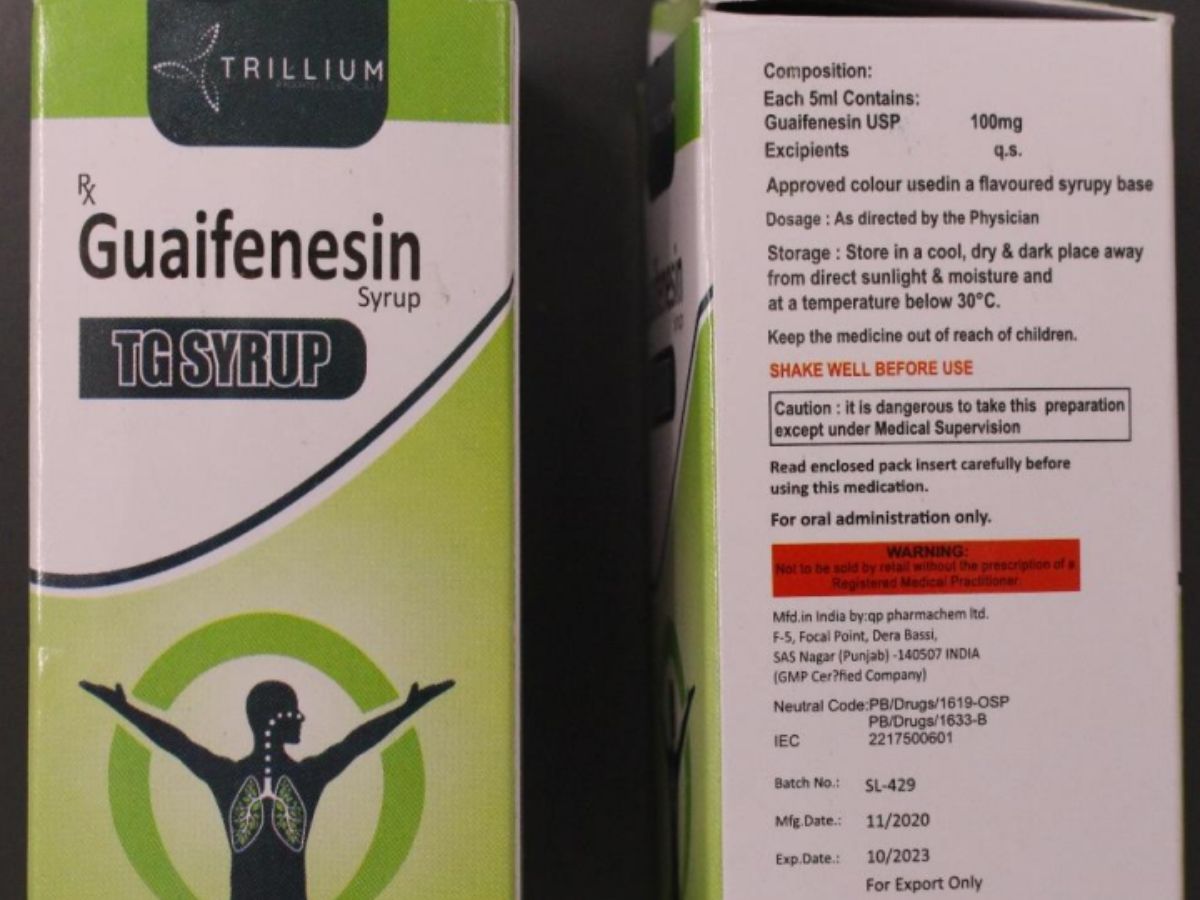
The World Health Organisation (WHO) on Tuesday issued an alert over a ‘contaminated’ syrup that is made and marketed by Indian Pharma Companies in Punjab and Haryana respectively. The UN body released a statement elaborating the usage of the syrup and what danger it poses to individuals who consume it. WHO also asked the authorities and health care professionals to look that no one uses the ‘substandard’ medicine.
“This WHO Medical Product Alert refers to a batch of substandard (contaminated) GUAIFENESIN SYRUP TG SYRUP identified in the Marshall Islands and Micronesia (Federated States of) and reported to WHO on 6 April 2023. Guaifenesin is an expectorant used to relieve chest congestion and the symptoms of cough,” the WHO said.
It added, “Samples of the GUAIFENESIN SYRUP TG SYRUP from the Marshall Islands were analysed by quality control laboratories of the Therapeutic Goods Administration (TGA) of Australia. The analysis found that the product contained unacceptable amounts of diethylene glycol and ethylene glycol as contaminants.”
“The stated manufacturer of the affected product is QP PHARMACHEM LTD (Punjab, India). The stated marketer of the product is TRILLIUM PHARMA (Haryana, India). To date, neither the stated manufacturer nor the marketer have provided guarantees to WHO on the safety and quality of these products,” WHO added further.
The alert went on to mention, “The product referenced in this Alert may have marketing authorizations in other countries in the Western Pacific region. It may have also been distributed, through informal markets, to other countries or regions.”
WHO Details Risks Of Taking The ‘Contaminated’ Syrup
The world health body said that Diethylene glycol and ethylene glycol are toxic to humans when consumed and can prove fatal. It added that the substandard product referenced in this Alert is unsafe and its use, especially in children, may result in serious injury or death. Toxic effects can include abdominal pain, vomiting, diarrhoea, inability to pass urine, headache, altered mental state and acute kidney injury which may lead to death.
READ FULL ALERT STATEMENT BY WHO HERE
Company's Reaction
Reacting to the alert issued by WHO on the syrup, the MD of its manufacturer, QP Pharma Chem Limited said that they doubt their product was ‘duplicated’.
Dera Bassi, Punjab | Food And Drug Administration of Punjab doubts that someone has duplicated the product (cough syrup) sent to Cambodia and then sold it in the Marshall Islands and Micronesia to defame the Government of India. The FDA department has taken samples of cough syrup… pic.twitter.com/t5H483Hc1Z
— ANI (@ANI) April 26, 2023
“Food And Drug Administration of Punjab doubts that someone has duplicated the product (cough syrup) sent to Cambodia and then sold it in the Marshall Islands and Micronesia to defame the Government of India. The FDA department has taken samples of cough syrup sent to Cambodia for testing. A total of 18,336 bottles of cough syrup were sent,” MD Sudhir Pathak told ANI.
http://dlvr.it/Sn50jZ
http://dlvr.it/Sn50jZ